SONG-PKD
Why develop core outcomes in polycystic kidney disease?
Polycystic kidney disease (PKD) affects approximately 12 million people worldwide. PKD is an irreversible life-threatening genetic disorder and is a major cause of end-stage kidney disease. Research in PKD aims to improve outcomes for patients yet they are rarely involved in outcome selection.
Identifying core outcomes
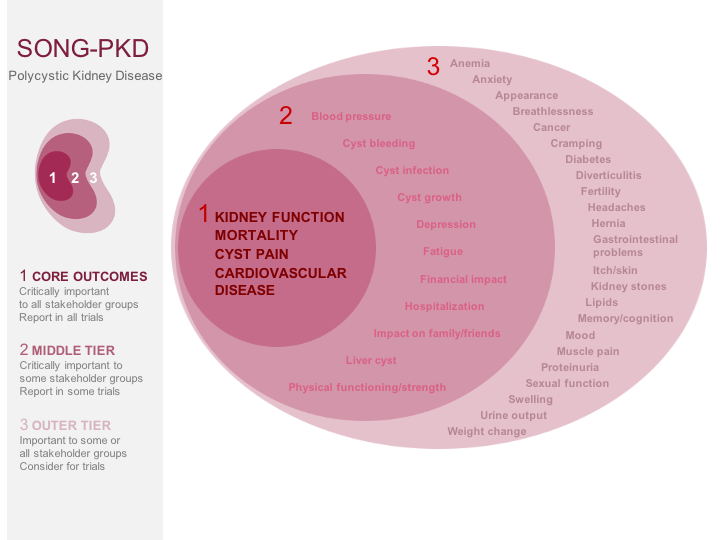
The standardised outcomes in nephrology – polycystic kidney disease (SONG-PKD) project will establish core outcomes that are based on the shared priorities of patients with PKD, their family members, clinicians, researchers, and policy makers. Eight focus groups using nominal group technique will be conducted with patients with PKD and their caregivers who will be asked to identify and rank outcomes that are important to them, and describe reasons underpinning their priorities. An international Delphi consensus survey will conducted with stakeholders, including patients, caregivers, and health professionals, to distil and generate a prioritised list of “core outcomes” to include in trials, other forms of research and clinical care.
SONG-PKD will establish a new core outcome set in PKD. This will help to ensure that research is measuring and reporting outcomes that are meaningful and relevant to patients with PKD, their family, and clinicians; to improve patient-centred care for people living with PKD.
SONG-PKD Steering Group
Gopala Rangan (Chair) | The University of Sydney, Australia
Albert Ong | University of Sheffield, United Kingdom
Arlene Chapman | University of Chicago, United States
Curie Ahn | Seoul National University Hospital, South Korea
Helen Coolican | PKD Foundation of Australia, Australia
Juliana Tze-Wah Kao | Fu Jen Catholic University Hospital, Taiwan
Kevin Fowler | Kidney Health Initiative, Patient Family Partnership Council; President, The Voice of the Patient, United States
Ron Gansevoort | University Medical Center Groningen, Netherlands
Ronald Perrone | Tufts Medical Center, United States
Tess Harris | PKD International, United Kingdom
Vicente Torres | Mayo Clinic, United States
York Pei | University of Toronto, Canada
Project coordinators: Yeoungjee Cho, University of Queensland; Talia Gutman, The University of Sydney; Benedicte Sautenet, University of Tours
Project updates
Protocol: The SONG-PKD protocol has been published in Trials.
Focus groups: We completed focus groups with patients and family members in Australia, France and South Korea. The manuscript has been submitted for publication.
Delphi survey: The SONG-PKD Delphi Survey is now closed. Thank you to everyone who participated!
SONG-PKD San Diego Workshop
The SONG-PKD Consensus Workshop was held on Thursday 25th October 2018. Patients with PKD, family members and health professionals came together to discuss the proposed core outcomes to be reported in all clinical trials in people with PKD. The discussions were extremely insightful and eye opening, particularly on the issues of pain, cardiovascular health, and cyst-related problems.